pH Levels Explained
When we talk about gardening or hydroponics, we often emphasise nutrients, light and water quality, but there's another key player that deserves our attention – pH. As someone who has spent years wading through the intricacies of horticulture with the Hyjo team, I can attest to how the pH level can make or break your plant's health. Simply put, pH is a measure of the acidity or alkalinity of a solution, indicated by a numeric scale that runs from 0 to 14. Pure water sits smack in the middle at 7, representing neutrality. Anything below 7 is acidic, while numbers above tilt towards alkaline or basic.
Understanding this seemingly tiny detail is crucial because each plant thrives in a certain pH range. It can affect everything from soil chemistry to nutrient availability. For instance, a high hydrogen ion concentration in acidic soils can hinder plants from accessing nitrogen, phosphorus, and potassium. Conversely, alkaline soils can limit the uptake of iron, manganese, and copper. Armed with a trusty pH meter, gardeners can gauge their growing medium's status and make necessary adjustments to optimise growth.
I'll walk you through what exactly impacts the shifting sands of your growing medium's pH and unpack how to manoeuvre through varying soil types - be it neutral, acidic, or alkaline. So whether you're wrestling with the pH range for coco media or navigating the waters of recirculating deep water culture systems, by the end of this, you'll be well-versed in tweaking your soil pH like a seasoned pro.
Key Takeaways
- pH measures the acidity or alkalinity of a solution on a scale from 0 to 14.
- Proper pH levels are crucial for nutrient availability and overall plant health.
- Understanding and adjusting soil pH is key to successful gardening and hydroponics.
What Is pH?
Have you ever wondered why some substances are sour to the taste like lemon juice, while others like baking soda have a completely different profile? Well, the answer often lies in a little thing we call pH. But what exactly is it?
pH stands for 'power of hydrogen' and is, quite simply, a measure of the hydrogen ion concentration in aqueous solutions. I think of pH as a covert agent working behind the scenes; its value can tell you if a solution is acidic, neutral, or alkaline. The term was first introduced by a Danish biochemist named Søren Peter Lauritz Sørensen.
The nitty-gritty of pH is more mathematical—it's the negative logarithm to the base 10 of the hydrogen ion concentration. This might sound a bit daunting, but it's a pretty straightforward concept. Solutions with more hydrogen ions are acidic, and those with fewer are alkaline.
Let's talk numbers. pH is measured on a logarithmic scale from 0 to 14. A pH of 7 is considered neutral—that's where pure water sits. Anything below 7 is acidic, like lemon juice (around pH 2) and vinegar (roughly pH 3). Above 7, you've got your alkaline substances; for instance, baking soda has a pH about 9, while bleach is way up there with a pH around 13.
This simple little scale isn't the worst place to start:
| pH Value | Common Substance | Classification |
|---|---|---|
| Around 2 | Lemon Juice | Acidic |
| Roughly 3 | Vinegar | Acidic |
| Exactly 7 | Pure Water | Neutral |
| About 9 | Baking Soda | Alkaline |
| Around 13 | Bleach | Alkaline |
pH doesn't just sit in the lab; it ventures out into the real world, affecting how nutrients are available in soil and influencing microorganisms and plant health. Something as simple as rainfall or the presence of organic matter can nudge the pH of natural soils in one direction or another.
So the next time you're tending to your garden or just enjoying a glass of something fizzy, remember that pH has a role in it all, even if it's just behind the scenes.
What Changes Growing Medium pH?
When I think about the vitality of plants, I often remember that the medium they grow in is much like a home to them. It's where they sip water and nutrients. But did you know this home, the growing medium, is subject to pH changes? Let's look at what causes these shifts, so we can keep our leafy friends chirpy!
Water Alkalinity
Alkalinity, not to be confused with pH, refers to water's capability to neutralise acidic substances. When the water you use is alkaline, it comes with calcium and magnesium ions in tow. These heroes can push the growing medium’s pH up. That’s why checking water quality with pH meters can save the day by letting us know if our water is more of a hero or a villain!
Fertiliser
Now, onto fertilisers, these can be a bit of a Jekyll and Hyde situation. Depending on what you use, they can release either acidic or basic ions. If the fertiliser's the acidic sort, it’ll lower the pH of the growing medium, swinging things towards a major stress for the plant. But if it's a basic chap, it lifts the pH, possibly making the environment too cushy for some plants. That's why knowing the right mix and amount is as important as the ingredients in my legendary Victoria sponge cake.
Crop
Lastly, crops are not just idle bystanders. They play an active role, too. Some are quite the personalities, taking up ions and causing a bit of a pH jumble. For example, as they pull up nutrients, they might leave hydrogen ions behind, which lowers the pH. It turns out that certain crops are more demanding than others, setting the tone for the growing medium's pH.
So, there we have it. We've talked water, fertiliser, and crops – all key players in the pH drama of a growing medium. Keep a close eye on these factors, and you won't be caught off guard when the pH starts to sway. Remember, a happy home means a happy plant, and a happy plant means a happier you! ????
pH Range for Coco Media
Have you ever wondered why plants in coco coir flourish? Well, let's take a peek into the world of soil pH and agronomy to understand this phenomenon. As a gardener myself, I operate within 5.7 to 6.4 ph range for optimum plant growth.
Coco coir, commonly used in horticulture, offers a unique environment for plant roots. Its natural pH range is about 5.8 to 6.5, which is slightly acidic and perfect for a diverse range of plants. If you're like me, obsessed with your plants' health, then paying attention to this aspect is crucial for nurturing a vibrant garden.
-
What's the big deal with pH?
Essentially, pH influences the soil's biome. The range I mentioned is prime real estate for beneficial microbial activity, which aids in nutrient uptake for your plants. -
Buffering Capacity
Coco coir has a remarkable ability to buffer, meaning it can maintain a stable pH, even when I make minor errors in feeding. This buffering capacity ensures my plants are less likely to suffer from sudden pH fluctuations.
Isn't it great when your growing medium works with you, rather than against you? To maintain this perfect pH balance, you might sometimes need to adjust the pH level by adding buffers. I regularly check the pH of my coco coir to ensure it stays within the ideal range for optimal plant health.
Given this specific medium's propensity to lean towards acidic conditions, every once in a while, I might have to nudge it back to the ideal range with a little pH up or down. But more often than not, if I keep my irrigation water balanced, my coco media does the heavy lifting, offering my plants a hospitable environment to grow to their fullest potential. Now that’s what I call a powerful partnership between gardener and ground!
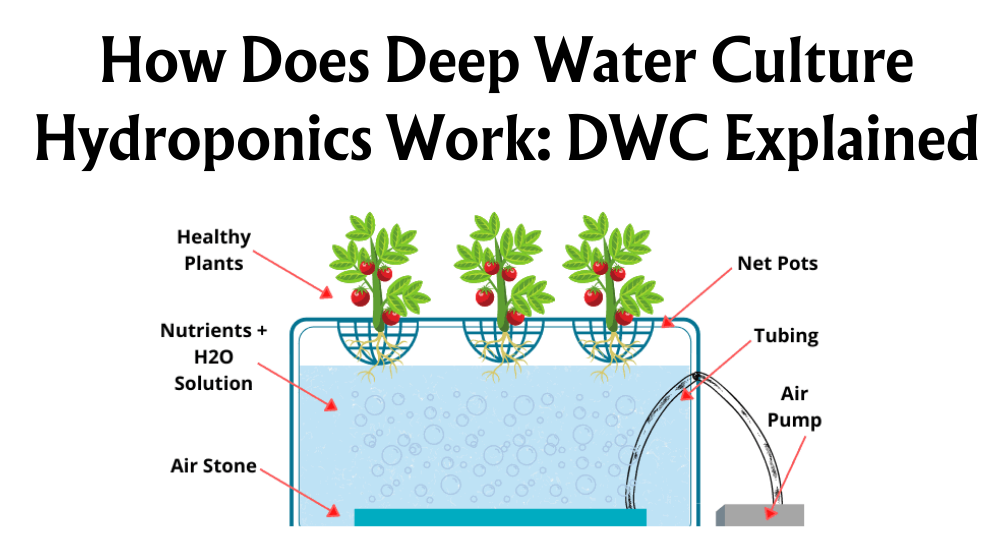
pH Range for RDCW Systems
Have you ever wondered about the secret sauce to thriving plants in a Recirculating Deep Water Culture (RDWC) system? I'll let you in on a little secret: it's all about the pH! Let's chat about why the pH range is critical for success in RDWC systems and how it links with other aspects, like water treatment and materials used for pipes, like copper.
In my experience running RDWC setups, keeping the water's pH between 5.8 and 6.2 has been the sweet spot for healthy plants. What's so magical about this range? Well, it keeps the nutrient solution in the perfect zone for optimal plant uptake.
Why this range? Plants are a bit fussy and like their environment just right. If the pH strays from our range, nutrients can either become unavailable (they effectively go into hiding) or too available (it's like a nutrient party, and everyone's invited), which causes an imbalance.
Quick Tip: A touch acidic is just perfect, mimicking the natural conditions many plants love.
Here's a nifty way to remember:
- 5.5 is too low — nutrient lockout will show!
- 6.5 is too high — nutrient toxicity might apply!
- 5.8 - 6.2 is just right — plants will grow strong and bright!
When it comes to water treatment, keeping pH under control helps to guarantee the longevity of my system. It prevents the buildup of minerals that can harm my plants or clog up the works. What's more, if you're using copper pipes, it's crucial to monitor the pH and maintain it within this range to prevent corrosion. The last thing we need is copper levels rising and putting the whole crop at risk!
Now, don't just set it and forget it. Regular checks and balances are key. I tend to test my water daily, just to be on the safe side. After all, prevention is better than cure, especially in the delicate ecosystem of RDWC. Grab yourself a trustworthy pH meter – your plants will thank you!
Alkaline Soils
Have you ever wondered why some plants in your garden thrive whilst others struggle, no matter what you do? The answer could lie beneath your feet, in the pH of the soil. So, what exactly are alkaline soils? Well, they're the ones that boast a pH above 7.0, indicating a soil that's more limestone chalk than lemon juice in character. These soils are rich in calcium and magnesium, and they often come with a few quirks that I'll talk you through.
What's the deal with these soils, then? On the plus side, they hang onto nutrients like a squirrel with a prized nut - less leaching, which is good news for plants. You won't find heavy metals causing a fuss either; they're far less toxic in these conditions. But here’s a ‘but’: essential nutrients such as iron and manganese become the shy types, less soluble and, thus, less available to plants. This can lead some plants to experience chlorosis, a fancy term for a sad, yellowing condition due to inadequate chlorophyll.
Now, not all plants are wallflowers at the alkaline soil party - some are the life and soul! Take lilac, honeysuckle, clematis, and lavender; they cope with the high pH by throwing their own adaptations into the mix, allowing them to flourish where others might flounder.
And if you're dealing with alkaline soils in your garden, don't fret. I’ve got some tips to help you manage:
- Choose Wisely: Pick plants that naturally like it on the higher side of the pH scale.
- Add Organic Matter: This can help improve nutrient availability and overall soil structure.
- Fertilise Smart: Utilise acid-forming fertilisers to nudge that pH down a bit.
- Avoid Lime and Wood Ash: They’re like fuel to the alkaline fire, so steer clear if you're trying to reduce alkalinity.
Overall, it's about working with what you've got and creating a haven for plants that can handle a bit of alkaline swagger in their step. Isn't it fascinating how a little scale from zero to fourteen can have such a big impact on our green-fingered pursuits?
Neutral Soils
Ever wondered what makes a soil 'just right' for a broad range of plants? Let's talk about neutral soils. Neutral soils have a pH that hovers around the 7.0 mark, which means they offer a sweet spot where plenty of nutrients are readily available to plants.
Why does this matter, you ask? Well, in this balanced environment, nutrient availability is at its best—think of it as a buffet where all the essential elements are up for grabs. Microbial activity is bustling, breaking down organic matter and boosting soil structure, which is vital for good drainage and aeration.
But let's not get carried away; even neutral soils have their quirks. They are very democratic, allowing a diverse array of plant species to thrive. This means gardeners like us have an easier time growing beauties like roses, sunflowers, tomatoes, and beans without having to fuss over adjusting the pH too much.
However, neutrality also comes with the risk of nutrient leaching—if it's easy come, it's easy go. Plus, with such an open invitation, plants might face more competition for those nutrients.
Maintaining this balance doesn't have to be a chore. I make it a point to:
- Test the soil pH regularly with a simple kit—I can't fix what I don't know.
- Add organic matter because it's like giving the soil a multivitamin.
- Choose balanced fertilizers to avoid swinging the pH pendulum too far one way or the other.
- Steer clear of extreme pH adjustments—it's all about harmony.
In the end, it's all about keeping it steady and avoiding major swings. That way, my garden remains a happy place for a whole range of plants to call home.
Acid Soils
Ever seen a garden radiant with rhododendrons or bouncy blueberries? What you've witnessed is the spectacular results of acid soils at work. So, what exactly are these soils? Acid soils are those with a pH lower than 7.0. I like to think of pH as the personality of the soil; below 7, and it's on the acidic side. Let's get into what this means for our green-thumbed pursuits.
Characteristics of Acid Soils:
- Low in Calcium and Magnesium: You'll find that these soils are often lacking in calcium and magnesium. It's a bit like a diet without dairy – some plants really struggle without these nutrients!
- High Iron and Manganese Availability: Iron and manganese have a field day in acid soils. They're more soluble, so plants can guzzle them up much easier.
- Challenging Structure: The structure might be poor, a bit like a crumbling cookie. Not the best environment for roots to thrive.
Pros and Cons for Plant Growth:
Here's the interesting bit – acid soils are something of a double-edged sword. They enhance the solubility of a few essential elements, which is brilliant! Plus, they tend to reduce disease incidence; fewer sick days for plants, yay! However, there's a catch. Acid soils can be a bit too much of a good thing – they bring toxicity into the equation, reduce microbial activity (think of microbes as the soil's workforce), and limit your plant choices quite drastically.
Plants That Love Acidic Conditions:
- Rhododendrons
- Camellias
- Heathers
- Blueberries
These botanical beauties have adapted to roll with the low pH punch. They've got survival down to a fine art, capable of thriving where others might wither.
Managing Acid Soils – My Tips for You:
- Choose Suitable Plants: Stick with plant pals that enjoy an acidic hangout.
- Lime or Dolomite Application: It's like an antacid for soil. It can help neutralise the excess acidity.
- Alkaline-forming Fertilisers: These are like bringing balance to a seesaw. They help to even things out.
- Avoid Sulfur and Peat Moss: They're the culprits when it comes to acidifying soil even further.
So, if you're blessed with this unique soil type, don't fret! I personally find acid soils fascinating, and with the right approach, you can curate a stunning garden that's both a haven for acidity-loving plants and a testament to your green-fingered skills.
Changing Your Soil pH
Ever wondered why your plants aren't thriving, even though you're giving them all the TLC they could ask for? The answer could lie just beneath your feet—in your soil pH. It's a bit like a dinner party for plants; the right pH sets the table, while the wrong one can disrupt the feast.
Testing soil pH is like a sneak peek at your garden's potential. Use a pH meter or test kit—super easy to find at your local garden centre, trust me. Knowing the current pH helps you avoid a gardening faux pas by ensuring you don't disturb the soil's acid-base balance needlessly.
If you're scratching your head wondering if you should tinker with the pH, consider these pointers. The golden rule? Only mess with the soil's pH if it's stopping your plants from grabbing their nutrients. The type of soil amendment—like lime to up the pH or sulphur to bring it down—depends on your starting point and where you want to end up. Think about dosh and effort too; plus, nobody fancies harming the environment with excess acid rain or alkalosis/acidosis situations!
Here's a rough guide to get your soil pH just right:
- To raise pH: A sprinkle of lime can work wonders. Picture this: tossing 5 kg of lime over a 100 m² garden could lift your pH by 0.5!
- To lower pH: A dash of elemental sulphur might be your new best friend.
You wouldn't pour salt without tasting your food first, would you? The same goes for soil amendments—small doses and a bit of patience do the trick. Keep an eagle eye on the pH over time, you don't want to tip your soil into a drama of over-liming or -sulfuring.
It may feel like a chemistry class but adjusting soil pH is really just gardening with a sprinkle of science. Now, go and get that pH 'just right' and watch your garden party bloom!
Conclusion
I've touched on several facets of pH in our daily lives, underlining its critical role. From the way plants absorb nutrients to the digestive process in our stomachs, it’s clear that the optimal pH level is key to health and functionality. Particularly for garden enthusiasts, understanding soil pH can be the difference between a thriving garden and a failing one.
Here's what I want you to remember:
- Soil pH is vital for plant growth. A balanced pH can improve nutrient availability and enhance microbial activity.
- Digestive system: Our stomachs rely on an acidic environment to break down food and ward off pathogens.
Practical steps for you:
- Test your soil's pH: This is a simple yet impactful way to ensure your plants receive the best care.
- Adjust pH accordingly: If your soil is too acidic or alkaline, it might be time to consider amendments like lime or sulfur.
I encourage you, especially if you love gardening, to roll up your sleeves and test your soil's pH. It's a small step that could lead to healthier, more vibrant plants. Who wouldn't feel a burst of pride looking at a lush, green garden that bears the fruits of their labour? Remember, your garden's success begins with understanding the foundation it’s built on—literally.





 Store Locator
Store Locator